Dolichospermum
Summary 3
Dolichospermum (formerly Anabaena) is a cyanobacteria genus that is commonly found in freshwater phytoplankton assemblages. In nutrient-rich lakes it can form dense blooms. Superficially, Dolichospermum and Anabaena look similar, but Dolichospermum can form gas vesicles, making it planktonic. Anabaena never forms gas vesicles and is usually associated with benthic environments. There are many common species of Dolichospermum, and more than one species may be present simultaneously in a toxic cyanobacteria bloom.
Description 3
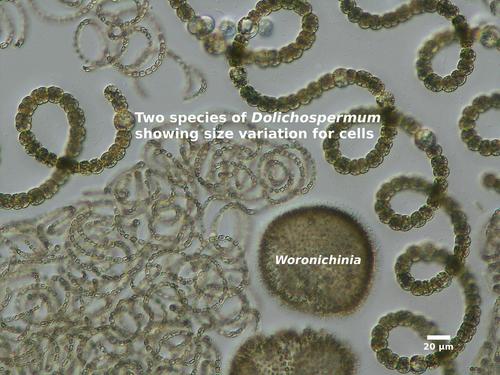
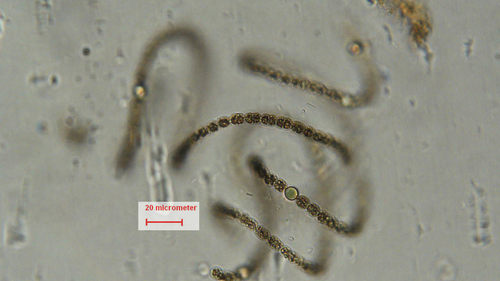
Individual Dolichospermum cells are spherical, oval, or barrel-shaped, and tiny (width = 5-20 μm; for comparison, a strand of spider silk is about 5 μm wide). Under magnification, Dolichospermum cells are dark brown and appear granular or mottled due to gas vesicles in the cells. (Anabaena, which does not form gas vesicles, will have cells that are pale blue-green or blue-gray in color.) The cells are joined together end-to-end to form long, unbranched filaments that are surrounded by clear, often transparent mucilage. Depending on the species, the filaments can be straight, bent, coiled, or irregularly twisted, and may be solitary or aggregated into tangled clumps.
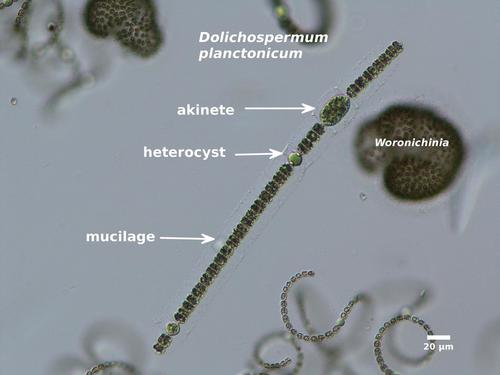
In addition to ordinary (vegetative) cells, the filaments may contain pale blue heterocytes (also called heterocysts) and large, granular, thick-walled akinetes. Heterocytes are specialized cells that convert dissolved nitrogen gas into ammonium that can be used for cell growth. Akinetes are resting cells that are resistant to cold temperatures and other unfavorable environmental conditions, and can overwinter in lake sediments. Heterocytes, and especially akinetes, are used to identify Dolichospermum (and Anabaena) to species.
Ecology 3
Dolichospermum blooms often form during warm, calm weather in lakes and ponds with relatively high nutrient concentrations (nitrogen or phosphorus) or low nitrogen to phosphorus ratios (N:P<15).
- Recent work suggests that high total phosphorus (TP) or total nitrogen (TN) concentrations are better predictors of bloom formation than N:P ratios.
- The gas vesicles in Dolichospermum cells provide a mechanism to move up and down in the water column, which increases access to nutrients and other growth factors.
Because Dolichospermum is capable of converting dissolved nitrogen gas ammonium, it can dominate blooms when inorganic nitrogen (ammonium, nitrate, and nitrite) is limiting to other types of algae.
- Nitrogen fixation requires a large amount of energy, so the relationship between nitrogen concentrations and Dolichospermum blooms is complicated; blooms can develop under both low and high inorganic nitrogen concentrations.
Dolichospermum blooms usually contain other types of Cyanobacteria, especially Aphanizomenon , Gloeotrichia, Microcystis, and Woronichinia .
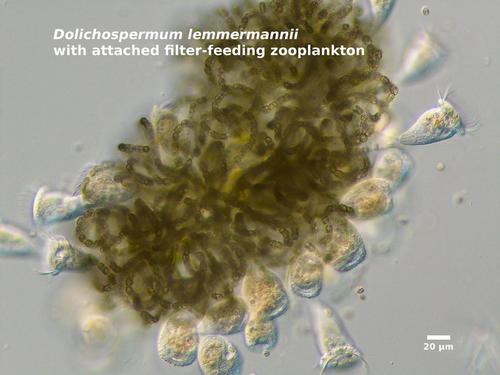
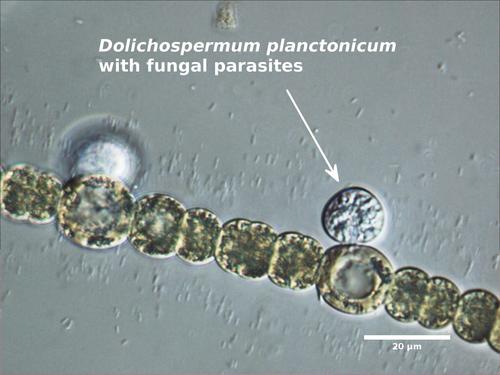
Although Dolichospermum filaments are rarely consumed by zooplankton, the cells are may be parasitized by viruses, bacteria, or fungi, and the tangled clumps of Dolichospermum may be colonized by stalked, filter-feeding zooplankton.
Toxicity 3
Identifying which cyanobacteria species are producing toxins is more difficult that it sounds. Historically, cyanobacteria taxa were described as "potentially" toxic based on whether they were collected in a toxic bloom. With the advancement of culturing techniques and genetic analysis, toxicity information is becoming more exact. But this is an ongoing process, so the toxicity information on these pages should be considered a work in progress.
Dolichospermum cells may produce microcystins (liver toxin), cylindrospermopsin (liver toxin), anatoxins (nerve toxin), saxitoxins (nerve toxin - paralytic shellfish toxin group), lipopolysaccharides (skin irritants), and BMAA (beta-Methylamino-L-alanine; nerve toxin). These toxins are released into the ambient environment when the cell wall is disrupted (cell lysis).
- Microcystins are rapidly degraded by naturally occurring but specialized bacteria. If the specialized bacteria are not present, microcystins can persist in the aquatic environment for months.
- Anatoxins are rapidly degraded by sunlight and at pH levels that are slightly above neutral (neutral pH = 7.0). At low pH levels, and in the absence of light, anatoxins may persist in the aquatic environment for a few weeks.
- There is some evidence that anatoxins can be degraded by specialized bacteria, similar to microcystins, but this process is not well documented.
- BMAA can bioaccumulate in zooplankton and fish, so this nerve toxin can contribute to health risks long after the toxic bloom has died back.
- There is not much information about environmental degradation of cylindrospermopsin and saxitoxins, but both types of toxins can persist for weeks in the aquatic environment.
Higher water temperatures and light appear to be associated with increased toxin production.
Not all Dolichospermum blooms result in the release of toxins.
Similar Genera 3
- Cyanobacteria: Anabaena, Cylindrospermum, Gloeotrichia, Nostoc , Woronichinia
- Other Algae: Batrachospermum (red algae), Geminella (green algae)
Information Sources 3
- Bennett, L. 2017. Algae, cyanobacteria blooms, and climate change. Climate Institute Report, April 2017.
- Berg, M and M. Sutula. 2015. Factors affecting the growth of cyanobacteria with special emphasis on the Sacramento-Jan Joaquin Delta. Southern California Coastal Water Research Project Technical Report 869.
- Caldwell Eldridge, S., R. Wood, and K. Echols. 2012. Spatial and temporal dynamics of cyanotoxins and their relation to other water quality variables in Upper Klamath Lake, Oregon, 2007-09. USGS Scientific Investigations Report 2012-5069.
- Chorus, I. and J. Bartram (Eds). 1999. Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. The World Health Organization E & FN Spon, London.
- D'Anglada, L., J. Donohue, J. Strong, and B. Hawkins. 2015. Health effects support document for the cyanobacterial toxin anatoxin-A. U.S. Environmental Protection Agency, Office of Water, EAP-820R15104, June 2015.
- EPA. 2014. Cyanobacteria and Cyanotoxins: Information for Drinking Water Systems. U. S. Environmental Protection Agency, Office of Water, EPA-810F11001.
- Graham, L. E., J. M. Graham, L. W. Wilcox, and M. E. Cook. 2016. Algae, Third Ed., ver 3.3.1 . LJLM Press, ww.ljlmpress.com.
- Granéli, E. and J. T. Turner (Eds.) 2006. Ecology of Harmful Algae. Ecological Studies, Vol. 189, Springer.
- Gury, M. D. and G. M. Guiry. 2017. AlgaeBase. world-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 10 November 2017.
- Lage, S., H. Annadotter, U. Rasmussen, and S. Rydberg. 2015. Biotransfer of B-N-Methlamino-L-alanine (BMAA) in a eutrophicated freshwater lake. Marine Drugs 13:1185-1201.
- Matthews, Robin A., "Freshwater Algae in Northwest Washington, Volume I, Cyanobacteria" (2016). A Collection of Open Access Books and Monographs. 6. http://cedar.wwu.edu/cedarbooks/6 (also see: http://www.wwu.edu/iws/).
- Meriluoto, J., L. Spoof, and G. Codd. 2017. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis. John Wiley & Sons, Chichester, UK.
- Paerl, H. W. 2014. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life 2014 4:988-1012.
- Wacklin, P., L. Hoffmann, and J. Komárek. 2009. Nomenclatural validation of the genetically revised cyanobacterial genus Dolichospermum (Ralfs ex Bornet et Flahault) comb. nova. Fottea 9:59-64.
- Walsby, A. E. 1994. Gas vesicles. Microbiological Reviews 58:94-144
Synonyms 3
Many species in the genus Anabaena have been moved to Dolichospermum. Revisions for some of the common species associated with toxic blooms are as follows:
- Dolichospermum circinale (formerly Anabaena circinalis).<\li>
- Dolichospermum crassum (formerly Anabaena crassa or Anabaena spiroides f. crassa).<\li>
- Dolichospermum flosaquae (formerly Anabaena flosaquae).<\li>
- Dolichospermum lemmermannii (formerly Anabaena lemmermannii or Anabaena flosaquae f. lemmermannii).<\li>
- Dolichospermum mendotae (formerly Anabaena mendotae).<\li>
Dolichospermum planctonicum (formerly Anabaena planctonica or Anabaena solitaria f. planctonica).<\li>
<\ul>
About 4
This guide was prepared by Dr. Robin Matthews, former Director of the Institute for Watershed Studies (http://www.wwu.edu/iws/) and professor emeritus at Western Washington University. In addition to this guide she has also written two ebooks (more on the way) on phytoplankton identification (see the "algae books" link on http://www.wwu.edu/iws/) and an online key to the cyanobacteria (http://www.snoringcat.net/cyanobacteria_key/index.html).
Sources and Credits
- (c) rmatth, some rights reserved (CC BY-NC-SA), uploaded by rmatth
- (c) John Bergeron, some rights reserved (CC BY-NC), uploaded by John Bergeron
- (c) rmatth, some rights reserved (CC BY-NC-SA)
- Adapted by rmatth from a work by (c) Bryan Milstead, some rights reserved (CC BY-NC-SA)